A Clinical Trial Design...

Highlights
- A clinical trial design that is often misunderstood is the #NonInferiority clinical trial design. 1/8
#MethodologyMonday (View Tweet)
- Mostly we set up trials to test if a new treatment is better than another (ie we test for superiority) but in a #NonInferiority design we wish to test if a treatment is not unacceptably worse than a comparator. 2/8
 (View Tweet)
(View Tweet)
- The main reasons why we might look for non-inferiority is when an alternative treatment is say much cheaper, or has fewer side effects … but we would only wish to use it if the benefits of the standard treatment are not significantly compromised. 3/8
https://t.co/lXXHam69ck
 (View Tweet)
(View Tweet)
- There are key elements to a non-inferiority design. You have to set the “non-inferiority margin”-how much worse can the alternative treatment be but still be acceptable.This requires careful consideration & needs input from all key stakeholders even if formal methods are used 4/8 (View Tweet)
- It is also important to note from the outset that you generally need a larger sample size to show something is non-inferior (compared to showing something is superior) 5/8 (View Tweet)
- Additionally, while in a superiority design an intention-to-treat analysis is usually the most conservative approach, this is often not the case in a non-inferiority trial - here a per-protocol analysis is usually more conservative. Both should show non-inferiority 6/8 (View Tweet)
- The following paper from the Trials journal also outlines many of the key issues with non-inferiority trials 7/8
https://t.co/PGjBHgU90i
 (View Tweet)
(View Tweet)
- Reporting of non-inferiority trials also requires special considerations. There is a dedicated CONSORT extension to help 8/8
see https://t.co/xdpFmujbm5
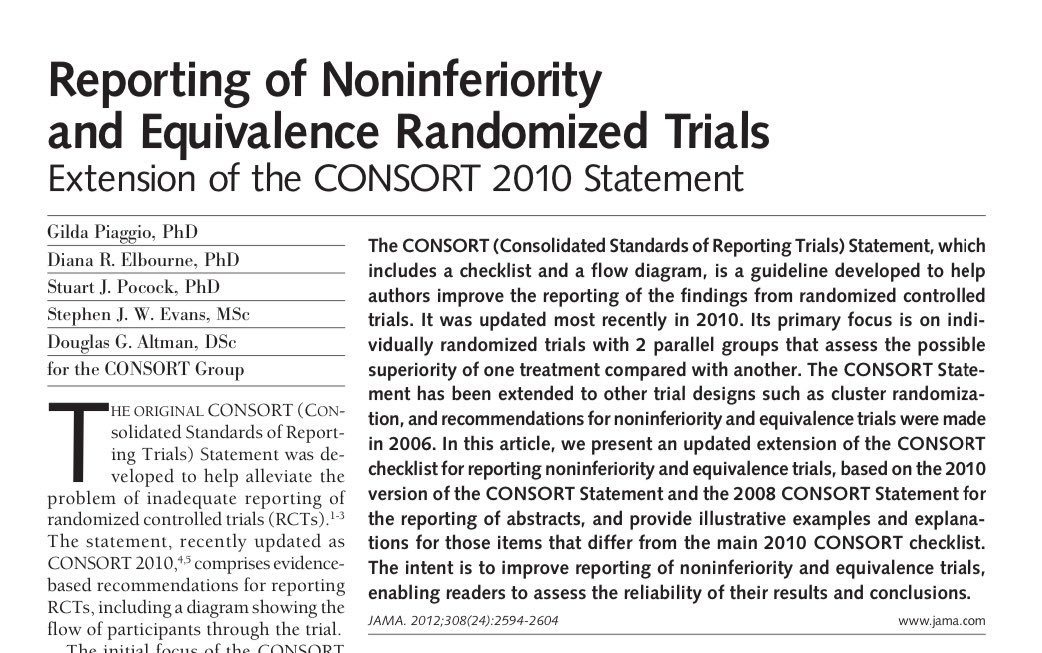 (View Tweet)
(View Tweet)

 (View Tweet)
(View Tweet) (View Tweet)
(View Tweet) (View Tweet)
(View Tweet) (View Tweet)
(View Tweet)